The peak values calculated in the bottom suitable panel of Figure 9B ended up around akin to People shown in the very best right panel of Determine 9A. Usually, after supply, the concentration of SPIONs from the aggregation location raises, along with the MPI detection sign really should be larger compared to homogeneous point out ahead of supply. Nevertheless, on shipping, the SPIONs were subjected to magnetic force, aggregated in shut proximity, and agglomerated, and the rise in particle dimensions resulted in minimized particle rotation, bringing about weakening with the MPI response signal. Hence, beneath the twin system of MPI sign enhancement by increasing focus and MPI signal reduction by particle clustering, the MPI signal just after supply remained in essence similar to prior to supply.
Whether the MPI sign boosts or decreases before and following shipping is affected from the parameters from the supply magnetic subject, the particle measurement of the SPIONs, the material of the coating layer, the surface area charge possible, the MPI magnetic subject parameters, along with other aspects, and the specific system on the effect should be confirmed by additional study. Nonetheless, it is certain that MPI technological know-how can be used to detect and image the aggregation condition and placement of magnetic medicine immediately after magnetic focusing on therapy.
collects the data of impurities and degradation profile of your drug compound throughout their development pathways. This allows a good deal in producing the method for separation of all doable impurities and degradation merchandise of targeted analyte.
A normal framework for method development based upon the analytical high quality by structure approach is presented and applied to the development of in the vicinity of-infrared spectroscopic methods. The framework is particularly compatible to safe stakeholder alignment, setting proper expectations and making sure that sources are expended correctly. Following location method aims and expectations and confirming feasibility, a danger assessment is done to establish many of the variables that might impact the method. The method is then designed While using the intention to mitigate the effect of People challenges. The result is a robust method that could be analyzed and validated if required with the regulatory ecosystem analytical method development of use.
Also, the main difference in radial magnetic subject power increases with variations in situation. Therefore, the therapeutic effect is somewhat various when the article for being examined is put in various radial positions while in the inner hole in the coil construction. The magnetic area parameters specifically have an affect on the movement and aggregation of magnetic medications, which consequently affect the effects of magnetic targeting therapy. Consequently, the design with the structure and parameters of your delivery coil is key to ensuring the success of magnetic concentrating on therapy.
If possible the movement rate is set not more than 2.0 mL/moment. The movement which provides the least retention moments, very good peak symmetries, least again pressures, and better separation of adjacent peaks/impurities can be the preferred being an optimized flow level for the Investigation.
Regretably, There is certainly minimal facts accessible in suggestions about regulatory anticipations pertaining to qualification compared with validation. It can be then part of the task sponsor's responsibility to ascertain its rationale for that analytical method lifecycle all through medical development.
Should you be a Sponsor looking for to run a scientific trial by way of a clinical investigate web site community, please contact us at data@sofpromed.com Clinical research plays a central role in advancing healthcare treatment plans and enhancing Health care results. To make certain The graceful...
Members on the BPT-A bunch and procedure development groups do the website job straight with each other and consistently exchange knowledge concerning the procedure and analytical results.
The movements of the two forms of SPION have been tested less than unique magnetic subject strengths and gradients. The alterations of their motion situations, determined by the magnetic industry toughness and gradient, had been observed to determine the required in vitro
Variations in vacancy get alter the lattice symmetry. This work shows how that may alter the electronic topology. And It appears very likely that vacancy buy can be used to induce topological modifications in other components in addition.”
Magnetic field parameters are essential in magnetic focusing on therapy. The delivery coils undoubtedly are a concrete realization of magnetic area parameters, and their framework and parameter style kind the core of magnetic drug focusing on therapy.
In case the sample preparing procedure involves different extraction steps to steer clear of the error within the extraction process, internal conventional technique shall be picked (Usually for derivatization approaches and bioanalytical methods).
Under the exact same gradient, as being the magnetic industry power increased, the common motion velocity of both SPIONs showed a craze of escalating and then stabilizing. Notably, the velocity inflection points of solitary-Main Nanoeast and multi-Main Resovist were being located at 270 mT and 260 mT, respectively, as proven in Figure 2A. Over-all, as the sphere toughness elevated, the velocity alteration of Nanoeast was better and faster, whereas that of Resovist tended to get comparatively sluggish.
 Judd Nelson Then & Now!
Judd Nelson Then & Now!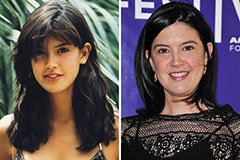 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Heather Locklear Then & Now!
Heather Locklear Then & Now! Erika Eleniak Then & Now!
Erika Eleniak Then & Now! Megyn Kelly Then & Now!
Megyn Kelly Then & Now!